Decoding DDX5: Understanding Male Fertility
The maintenance of sperm production is tightly controlled and coordinated, with a number of molecular mediators involved in the process. New research from ARMI has found that DDX5 is vital for male fertility, outlining the multifaceted role this protein has.
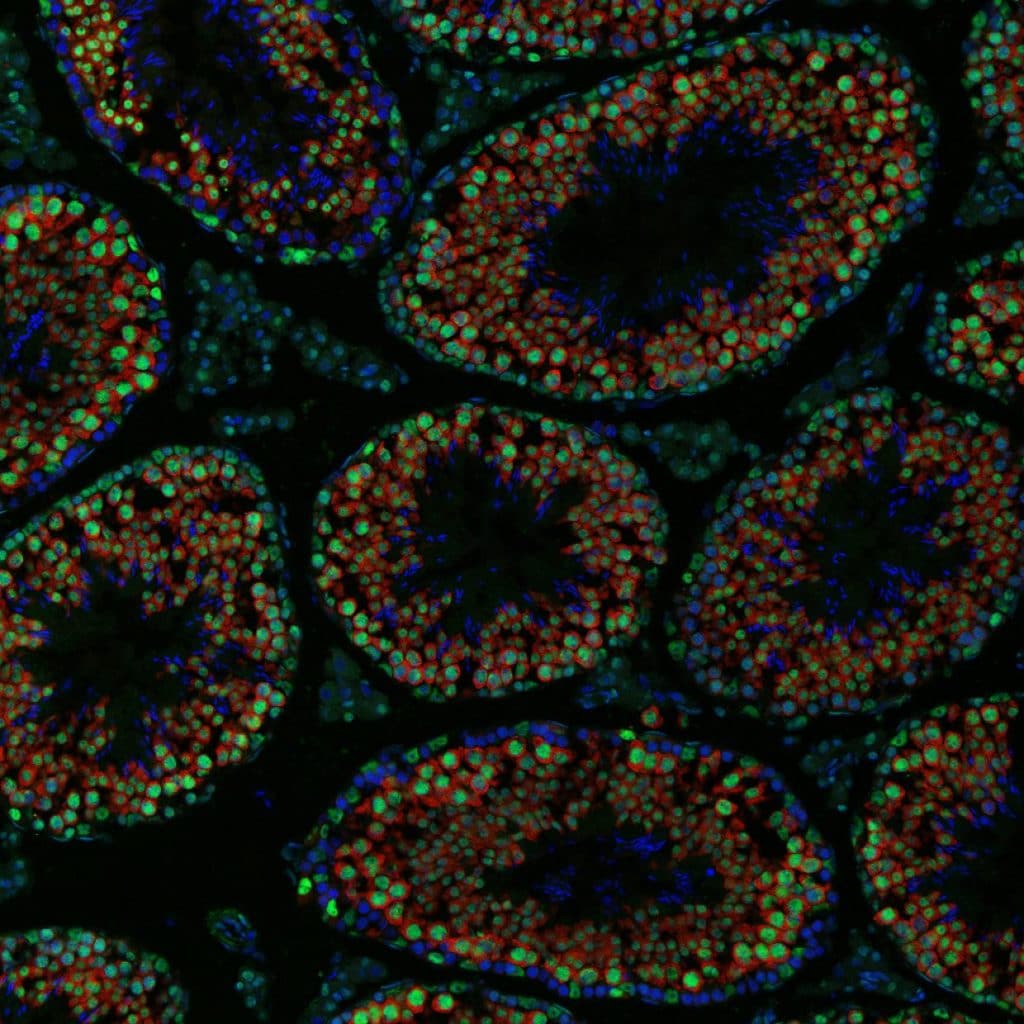
Adult mouse seminiferous tubule cross-section showing nuclear expression of DDX5 in all VASA-positive germ cells.
It’s always busy at the lab benches of ARMI, but it has been even more so lately, with Dr Julien Legrand, his colleagues from the Hobbs group and collaborators having had their work published in Nature Communications. Their research revealed that “DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia.”
“Our research unveils a multi-faceted key player, DDX5, in the maintenance and function of spermatogonia, which is a specific type of undifferentiated cell produced during an early stage in the formation of sperm cells. Spermatogonia is critical to sperm production and yet there is so much we don’t understand about how these cells are regulated,” commented Dr Legrand.
“Our data uncovers an essential role for DDX5 within undifferentiated and differentiating spermatogonia, and characterises DDX5 as a critical regulator of male fertility. This work helps us understand not only the complex pathways and key factors involved in health sperm production, but also the potential culprits causing male infertility as well as and potential therapeutic targets.” Dr Julien Legrand.
In an international collaboration that stretched from ARMI to the UK, Research Fellow Dr Legrand spearheaded the project using an inducible knockout mouse model and a range of sophisticated molecular biology, imaging, and gene and protein expression techniques.
The maintenance of sperm production is tightly controlled and coordinated, with a number of external and internal factors affecting the process. The paper shows that DDX5, a RNA helicase protein, is vital for male fertility. Affecting both gene and protein expression, the paper outlines DDX5’s role in regulating the gene splicing of key genes necessary for sperm production, in regulating the expression of cell cycle genes that are critical for cell proliferation and survival, and in being involved as a co-activator in the regulation of select target genes.
“Our data uncovers an essential role for DDX5 within undifferentiated and differentiating spermatogonia, and characterises DDX5 as a critical regulator of male fertility. This work helps us understand not only the complex pathways and key factors involved in health sperm production, but also the potential culprits causing male infertility as well as and potential therapeutic targets,” said Dr Legrand.
In addition to this, this research may have implications beyond reproductive biology, as sperm production is an ideal model for the study of adult stem cells. As such, identifying the important factors and mechanisms governing this process may have downstream impacts on research that uses adult stem cells as well as treatments.
Once again, congratulations Dr Legrand and colleagues!
More information
Click here to read the publication: Legrand, J. M. D. et al. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nature Communications 10, 2278, doi:10.1038/s41467-019-09972-7 (2019).
Dr Julien Legrand is a Research Fellow in the Hobbs Group, led by Dr Robin Hobbs. The primary research aim of the Hobbs Group is to identify and define the critical molecular mechanism underlying adult stem cells by using germline stem cells from the testis as a model system. The Hobbs group aims to uncover the self-renewal capabilities of adult stem cells – this will be important to the fields of fertility, tissue regeneration and cancer. For more information on Dr Robin Hobbs and his group at ARMI, please visit the Hobbs Group page. You can contact Dr Robin Hobbs via robin.hobbs@monash.edu. You can contact Dr Julien Legrand via julien.legrand@monash.edu or follow him on Twitter @ResearchJulien.
