New research highlights crucial protein-protein interactions in muscle development
A discovery from the Currie Group at the Australian Regenerative Medicine Institute (ARMI), recently published in the high-impact journal Cell Reports, has shown a crucial role for TCP-1 ring complex (TRiC) in the formation of skeletal muscle and the hereditary neuromuscular disorder, nemaline myopathy. This work, led by Senior Research Fellow Dr Joachim Berger, provides novel insights into the genetic and molecular intricacies of muscle development.
‘By understanding the interplay between proteins during this important process and their impact on a grander scale, we can begin to uncover particular steps where we can intervene. This could potentially lead to new therapies for the myriad of diseases in which TRiC is involved, such as Parkinson’s and Huntington’s disease,’ stated Dr Berger.
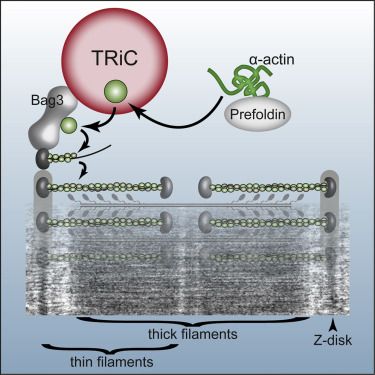
TRiC is a complex that enables proteins to obtain their correct shape, which is vital for the proper function of proteins. It has been estimated that TRiC folds 5-10% of all mammalian proteins, including actin, a well-studied and significant component of the muscle’s contractile apparatus. Previous research has led to the notion that TRiC is absolutely required for actin to obtain its three-dimensional structure.
For the first time, Dr Berger and his collaborators have studied the role of TRiC in living animals and demonstrated that, in contrast to the current notion, TRiC is not “absolutely required” for actin folding. This research has shown exactly where TRiC acts in muscle cells and how its function affects actin. By introducing specific mutations into the TRiC protein complex, the function of this protein was disrupted, which led to inefficient and impaired muscle development, skeletal muscle defects and muscle weakness. In line with these results, it was also found that TRiC function was involved in nemaline myopathy, a disorder characterised by abnormal actin aggregates.
The publication was the result of both national and international collaborations, involving researchers from:
- Australian Regenerative Medicine Institute
- EMBL Australia
- The University of New South Wales
- Victor Chang Cardiac Research Institute
- Karolinska Institutet, Sweden
These findings demonstrate the delicate relationship between protein function, normal development and downstream defects. ‘Our in vivo research has revealed the key role that TRiC plays in the folding of actin. Accordingly, TRiC is also crucial in facilitating the pathological effect of mutated actin. With this, we are only beginning to uncover its importance in skeletal muscle development and in muscle-related disorders,’ said Dr Berger.
The Cell Reports paper is available online.
For more information on Dr Joachim Berger and the Currie group at ARMI please visit the Currie Group page.
Contact information
Dr Joachim Berger
Senior Research Fellow
Phone: +61 (3) 9902 9621
Email: joachim.berger@monash.edu
Notes to Editors About ARMI
The Australian Regenerative Medicine Institute (ARMI) is dedicated to unlocking the regenerative capabilities of the human body. ARMI is a medical research centre based at the Clayton Campus of Monash University. Boasting 15 research groups studying a variety of regenerative approaches, ARMI is one of the largest regenerative medicine and stem cell research hubs in the world.
