How pluripotent cells transform into diverse cell types forming complex tissues is one of the most fascinating and most important question in biology. Pivotal advances were made recently on the genetic and epigenetic level, and yet the mystery remains how cell fate decisions are regulated by understanding the cell biology of pluripotent cells – in space and time, under physiological 3D conditions and at the cellular and subcellular level. To unravel how the microtubule cytoskeleton controls cell state transitions, the Zenker group uses innovative live imaging technologies to visualize the development of the living preimplantation mouse embryo and formation of human induced pluripotent stem cells (hiPSCs).
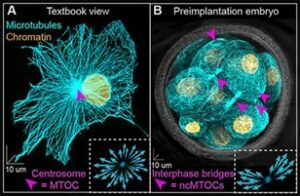
Figure 1. Microtubule diversity. (A) Centrosomal and (B) embryo-specific non-centrosomal microtubule organizing centers (pink arrowheads). (Zenker et al., 2017, Science)
Inside a cell, organelles and proteins are usually not randomly distributed but assigned to regions where they are needed. The cell utilises a network of filament-like structures, the microtubule cytoskeleton, as a road map to localise organelles and to trigger the relay of signals intra- and intercellularly. Work of the Zenker Lab are the first to demonstrate the importance of the microtubule network during early mammalian embryogenesis (Zenker et al., 2017, Science; Zenker et al., 2018, Cell; Hawdon et al., 2021, Development; Greaney et al., 2021, JoVE) which was until then widely regarded as disorganised and its contribution to cell fate specification was largely ignored.
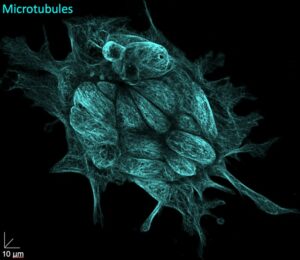
Figure 2. Discovering the unknown by using innovative imaging. High spatial resolution imaging of a hiPSC colony fluorescently labelled for the microtubule cytoskeleton.
Similar to the pluripotent cells of the living embryo, hiPSCs have the distinguished ability to differentiate into any cell type of the adult body. Like marking a needle in a haystack, looking into the inside of living pluripotent stem cells will make it possible to simply spot true pluripotent stem cells within a cluster of cells, but also to rectify the subcellular organisation by developing innovative non-invasive light-inducible microtubule drugs. Our work will enable us to significantly refine the current technologies to generate and select successfully reprogrammed cells for regenerative applications.

Group Members








Research Themes
- Unravelling the microtubule real-time organisation of pluripotent cells using live imaging
- Microtubule-dependent stem cell plasticity orchestrated by centrosomal and non-centrosomal switching
- To engineer non-invasive light-inducible tools to open up new ways to manipulate the microtubule cytoskeleton with spatiotemporal precision
- To repurpose our discoveries for the application of human induced pluripotent stem cells (hiPSCs) in regenerative medicine
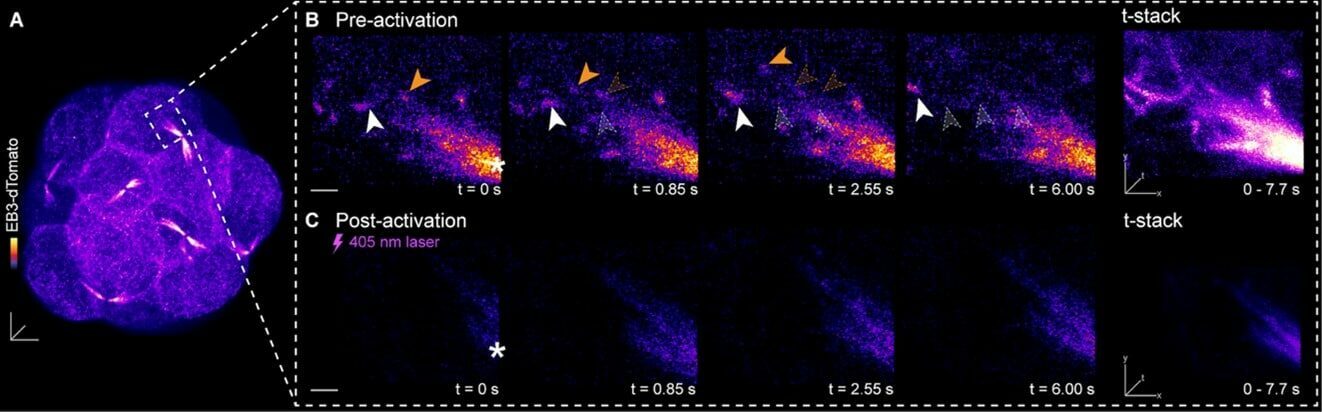
Figure 3. Microtubule drugs coming into light. (A) 3D living preimplantation mouse embryo, labelled for microtubule plus end marker EB3-dTomato. (B, C) Region of non-centrosomal microtubule organising centre, tracking EB3-dTomato comets (B) before and (C) after subcellular UV light activation of light-inducible microtubule growth inhibitors in the living mouse embryo. (Greaney et al., 2021, JoVE)
Featured Publications
| Authors | Title | Published In |
|---|---|---|
Hawdon, A., Geoghegan, N.D., Mohenska, M., Elsenhans A., Ferguson C., Polo J.M., Parton R.G., Zenker J. |
Apicobasal RNA asymmetries regulate cell fate in the early mouse embryo. |
Nat Commun 14, 2909 (2023). https://doi.org/10.1038/s41467-023-38436-2 |
Liu X, Tan JP, Schröder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G, Zhou Y, Poppe D, Lister R, Clark AT, Rackham OJL, Zenker J, Polo JM. |
Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. |
Nature. 2021 Mar;591(7851):627-632. doi: 10.1038/s41586-021-03372-y. Epub 2021 Mar 17. PMID: 33731926. |
Zenker J,* White MD*, Gasnier M*, Alvarez YD, Lim HYG, Bissiere S, Biro M, Plachta N. |
Expanding Actin Rings Zipper the Mouse Embryo for Blastocyst Formation. |
Cell. 2018 Apr 19;173(3):776-791.e17. doi: 10.1016/j.cell.2018.02.035. Epub 2018 Mar 22. |
Zenker J, White MD, Templin RM, Parton RG, Thorn-Seshold O, Bissiere S, Plachta N. |
A microtubule-organizing center directing intracellular transport in the early mouse embryo. |
Science. 2017 Sep 1;357(6354):925-928. doi: 10.1126/science.aam9335. |
More Publications
| Authors | Title | Published In |
|---|---|---|
Liu L, Neve M, Perlaza-Jimenez L, Xi X, Purcell J, Hawdon A, Conn SJ, Zenker J, Tamayo P, Goodall GJ, Rosenbluh J. |
Systematic loss-of-function screens identify pathway-specific functional circular RNAs |
Nat Cell Biol (2024). https://doi.org/10.1038/s41556-024-01467-y |
Jin H, Han Y, Zenker J. |
Cellular mechanisms of monozygotic twinning: clues from assisted reproduction. |
Human Reproduction Update, dmae022, doi.org/10.1093/humupd/dmae022. |
Stathatos GG, Merriner DJ, O’Connor A, Zenker J, Dunleavy JEM, O’Bryan MK. |
Epsilon tubulin is an essential determinant of microtubule-based structures in male germ cells. |
EMBO Rep (2024) 25: 2722 – 2742 |
Zenker, J. |
Traffic lights on early embryo development help understand cell pluripotency. |
Research Features, 147. (2023) |
Palacios Martínez S, Greaney J, Zenker J. |
Beyond the centrosome: The mystery of microtubule organising centres across mammalian preimplantation embryos. |
Curr Opin Cell Biol. (77), 102114, doi.org/10.1016/j.ceb.2022.102114 (2022). |
Greaney J, Hawdon A, Stathatos G, Aberkane A, Zenker J. |
Spatiotemporal Subcellular Manipulation of the Microtubule Cytoskeleton in the Living Preimplantation Mouse Embryo using Photostatins. |
J. Vis. Exp. (177), e63290, doi:10.3791/63290 (2021). |
Hawdon A, Aberkane A, & Zenker J. |
Microtubule-dependent subcellular organisation of pluripotent cells. |
Development, 148(20), dev199909. |
Stathatos GG, Dunleavy JEM, Zenker J, O’Bryan MK. |
Delta and epsilon tubulin in mammalian development. |
Trends Cell Biol. 2021 Apr 15:S0962-8924(21)00066-0. doi: 10.1016/j.tcb.2021.03.010. Epub 2021 Apr 15. PMID: 33867233. |
White MD, Zenker J, Bissiere S, Plachta N. |
Instructions for Assembling the Early Mammalian Embryo.
|
Dev Cell. 2018 Jun 18;45(6):667-679. doi: 10.1016/j.devcel.2018.05.013. |
Zhao ZW*, White MD*, Zenker J*, Alvarez YD, Bissiere S, Plachta N. |
Quantifying transcription factor-DNA binding in single cells in vivo with photoactivatable fluorescence correlation spectroscopy. |
Nat Protoc. 2017 Jul;12(7):1458-1471. doi: 10.1038/nprot.2017.051. Epub 2017 Jun 29. |
Stettner M, Zenker J, Klingler F, Szepanowski F, Hartung HP, Mausberg AK, Kleinschnitz C, Chrast R, Kieseier BC. |
The role of Peripheral Myelin Protein 2 in Remyelination.
|
Cell Mol Neurobiol. 2018 Mar;38(2):487-496. doi: 10.1007/s10571-017-0494-0. Epub 2017 Apr 26. |
White MD, Zenker J, Bissiere S, Plachta N. |
How cells change shape and position in the early mouse embryo.
|
Current Opinion in Cell Biology 2017 Feb;44:7-13. doi: 10.1016/j.ceb.2016.11.002. Epub 2016 Dec 26. |
Barneo-Muñoz M, Juárez P, Civera-Tregón A, Yndriago L, Pla-Martin D, Zenker J, Cuevas-Martín C, Estela A, Sánchez-Aragó M, Forteza-Vila J, Cuezva JM, Chrast R, Palau F. |
Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of charcot-marie-tooth neuropathy.
|
PLoS Genet. 2015 Apr 10;11(4):e1005115. doi: 10.1371/journal.pgen.1005115. eCollection 2015 Apr. |
Zenker J, Stettner M, Ruskamo S, Domènech-Estévez E, Baloui H, Médard JJ, Verheijen MH, Brouwers JF, Kursula P, Kieseier BC, Chrast R. |
A role of peripheral myelin protein 2 in lipid homeostasis of myelinating Schwann cells. |
Glia. 2014 Sep;62(9):1502-12. doi: 10.1002/glia.22696. Epub 2014 May 21. |
Ngo L, Haas M, Qu Z, Li SS, Zenker J, Teng KS, Gunnersen JM, Breuss M, Habgood M, Keays DA, Heng JI. |
TUBB5 and its disease-associated mutations influence the terminal differentiation and dendritic spine densities of cerebral cortical neurons.
|
Hum Mol Genet. 2014 Oct 1;23(19):5147-58. doi: 10.1093/hmg/ddu238. Epub 2014 May 15. |
Zenker J, Ziegler D, Chrast R. |
Novel pathogenic pathways in diabetic neuropathy.
|
Trends Neurosci. 2013 Aug;36(8):439-49. doi: 10.1016/j.tins.2013.04.008. Epub 2013 May 29. |
Azzedine H, Zavadakova P, Planté-Bordeneuve V, Vaz Pato M, Pinto N, Bartesaghi L, Zenker J, Poirot O, Bernard-Marissal N, Arnaud Gouttenoire E, Cartoni R, Title A, Venturini G, Médard JJ, Makowski E, Schöls L, Claeys KG, Stendel C, Roos A, Weis J, Dubourg O, Leal Loureiro J, Stevanin G, Said G, Amato A, Baraban J, LeGuern E, Senderek J, Rivolta C, Chrast R. |
PLEKHG5 deficiency leads to an intermediate form of autosomal-recessive Charcot-Marie-Tooth disease. |
Hum Mol Genet. 2013 Oct 15;22(20):4224-32. doi: 10.1093/hmg/ddt274. Epub 2013 Jun 17. |
Zenker J, Poirot O, de Preux Charles AS, Arnaud E, Médard JJ, Lacroix C, Kuntzer T, Chrast R. |
Altered distribution of juxtaparanodal kv1.2 subunits mediates peripheral nerve hyperexcitability in type 2 diabetes mellitus.
|
J Neurosci. 2012 May 30;32(22):7493-8. doi: 10.1523/JNEUROSCI.0719-12.2012. |
Stendel C, Roos A, Kleine H, Arnaud E, Ozçelik M, Sidiropoulos PN, Zenker J, Schüpfer F, Lehmann U, Sobota RM, Litchfield DW, Lüscher B, Chrast R, Suter U, Senderek J. |
SH3TC2, a protein mutant in Charcot-Marie-Tooth neuropathy, links peripheral nerve myelination to endosomal recycling.
|
Brain. 2010 Aug;133(Pt 8):2462-74. doi: 10.1093/brain/awq168. |
de Preux Charles AS, Verdier V, Zenker J, Peter B, Médard JJ, Kuntzer T, Beckmann JS, Bergmann S, Chrast R. |
Global transcriptional programs in peripheral nerve endoneurium and DRG are resistant to the onset of type 1 diabetic neuropathy in Ins2 mice.
|
PLoS One. 2010 May 26;5(5):e10832. doi: 10.1371/journal.pone.0010832. |
Arnaud E, Zenker J, de Preux Charles AS, Stendel C, Roos A, Médard JJ, Tricaud N, Kleine H, Luscher B, Weis J, Suter U, Senderek J, Chrast R. |
SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system.
|
Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17528-33. doi: 10.1073/pnas.0905523106. Epub 2009 Sep 29. |




